Hg–C bond protonolysis by a functional model of bacterial enzyme organomercurial lyase MerB†
Abstract
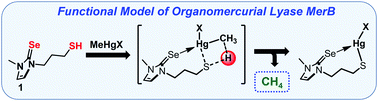
Herein, we report a novel synthetic compound 1, having a highly nucleophilic selenolate (Se−) moiety and a thiol (–SH) functional group, which showed efficient Hg–C bond protonolysis of various R–Hg–X molecules including neurotoxic methylmercury and thimerosal, via direct –SH proton transfer to the highly activated C-atom of a departed R group with low activation energy barrier at room temperature (21 °C), in the absence of any external proton source and, thus, acts as a functional model of MerB.


 Please wait while we load your content...
Please wait while we load your content...