Regio- and diastereoselective Pd-catalyzed synthesis of C2-aryl glycosides†
Abstract
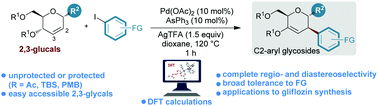
An efficient regio- and diastereoselective arylation method of readily available 2,3-glycals with various aryl iodides has been established. Using the Pd(OAc)2/AsPh3 precatalytic system, this protocol proved to be general to prepare a variety of substituted C2-aryl glycosides in good yields with complete diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...