Superelectrophilic Csp3–H bond fluorination of aliphatic amines in superacid: the striking role of ammonium–carbenium dications†
Abstract
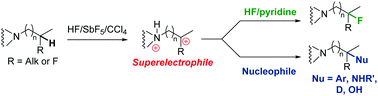
The superacid-promoted electrophilic Csp3–H bond activation of aliphatic amines generates superelectrophilic species that can be subsequently fluorinated. Demonstrated by low-temperature in situ NMR experiments, the ammonium–carbenium dications, crucial for this reaction, can also react with C–H bonds opening future synthesis perspectives for this mode of activation.


 Please wait while we load your content...
Please wait while we load your content...