Organocatalytic enantioselective allylic alkylation of α-aryl γ-lactones: an approach to densely functionalized quaternary stereocentres†
Abstract
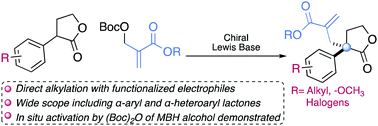
The asymmetric allylic alkylation (AAA) of α-aryl γ-lactones involving the activation of Morita–Baylis–Hillman (MBH) carbonates by an original chiral Lewis base is reported. A wide range of γ-lactones bearing a quaternary stereocentre was thus obtained in both high yields and high enantiomeric ratios. The direct alkylation by MBH alcohol using in situ activation has been also established. Additionally synthetically useful functional transformations of groups surrounding the quaternary stereocentre have been performed.


 Please wait while we load your content...
Please wait while we load your content...