Synthesis of benzyl sulfides via substitution reaction at the sulfur of phosphinic acid thioesters†
Abstract
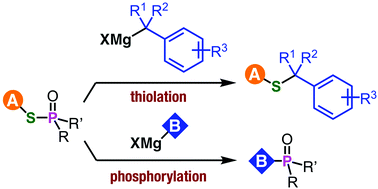
An ambident electrophilicity of phosphinic acid thioesters is disclosed. Unexpected carbon–sulfur bond formation took place in the reaction between phosphinic acid thioesters and benzyl Grignard reagents. The developed method for benzyl sulfides has a wide substrate scope and was applicable for the synthesis of a drug analog.


 Please wait while we load your content...
Please wait while we load your content...