Drastic fluorine effect: complete reversal of the selectivity in the Au-catalyzed hydroalkoxylation reaction of fluorinated haloalkynes†
Abstract
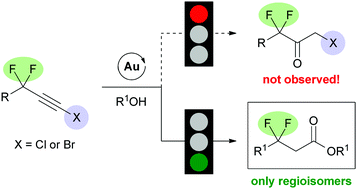
The gold-catalyzed hydration reaction of haloalkynes is highly regioselective producing 2-halomethylketones as the sole products. Herein, we document a drastic fluorine effect where the reaction of 1-halo-3,3-difluoroalkynes as substrates leads to a complete reversal of selectivity and produces 3,3-difluoroesters as the unique products.


 Please wait while we load your content...
Please wait while we load your content...