Diprotonative stabilization of ring-opened carbocationic intermediates: conversion of tetrahydroisoquinoline to triarylmethanes†
Abstract
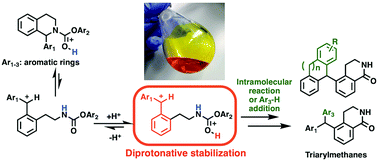
Superacid-promoted conversion of tetrahydroisoquinolines to triarylmethanes via tandem reactions of C–N bond scission, Friedel–Crafts alkylation, C–O bond scission, and electrophilic aromatic amidation was developed. Dication formation was important for stabilizing the ring-opened carbocationic intermediate, which is a new role for diprotonation in reaction mechanisms.


 Please wait while we load your content...
Please wait while we load your content...