Synthesis of functionalized tetrahydropyrans via cascade cycloaddition involving silyloxyallyl cation intermediates†
Abstract
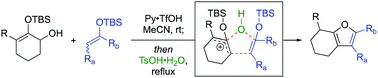
An expedient synthesis of highly substituted tetrahydrobenzofuran via an unsymmetrical silyloxyallyl cation is reported. Conveniently generated under catalytic Brønsted acid conditions, nucleophilic capture of this reactive intermediate by silylenolate, followed by Paal–Knorr cascade cyclization in the presence of tosic acid monohydrate effectively constructed the tetrahydrobenzofuran core in a single synthetic step.


 Please wait while we load your content...
Please wait while we load your content...