Catalytic cycle of carbohydrate dehydration by Lewis acids: structures and rates from synergism of conventional and DNP NMR†
Abstract
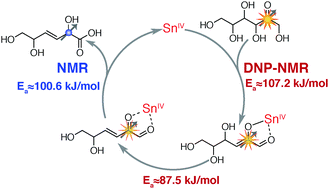
Lewis acids play key roles in many chemical reactions. Structural and functional (kinetic) detail in Lewis acid catalysed fructose conversion are derived herein by the combined use of conventional and dissolution dynamic nuclear polarization (D-DNP) NMR. Structural information obtained with D-DNP NMR was used to identify conditions that stabilize an elusive initial intermediate and to determine its chemical structure. Carbohydrate dehydration through this intermediate had been predicted computationally. Complementary kinetic NMR assays yielded rate constants spanning three orders of magnitude for the three biggest energy barriers in the catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...