Rotation-restricted thermally activated delayed fluorescence compounds for efficient solution-processed OLEDs with EQEs of up to 24.3% and small roll-off†
Abstract
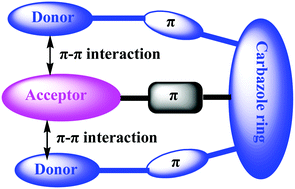
Two triphenylamine or 4,4′-di(tert-butyl)triphenylamine groups are introduced at the 1,8-positions of 3,6-di(tert-butyl)-9-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)carbazole to yield two emitters containing a cofacial donor–acceptor–donor chromophore, which exhibit strong TADF characteristics dominated by through-space charge-transfer. The solution-processed OLEDs achieve maximum external quantum efficiencies of up to 17.4% and 24.3% with small efficiency roll-off rates.


 Please wait while we load your content...
Please wait while we load your content...