Storage and release of two electrons from an electron-rich carbon–carbon bond: boron mediated reversible coupling of DMAP and 9-azajulolidine†
Abstract
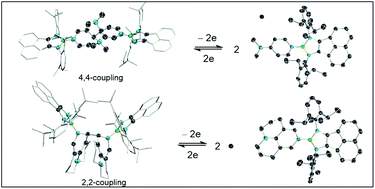
This manuscript describes the preparation of [{(dpp-bian)BBr}Li(DME)3] (2, dpp-bian = 1,2-bis[(2,6-diisopropylphenyl) imino]acenaphthene) and [{(dpp-bian)BL}+Br−] (5, L = DMAP; 6, L = 9-azajulolidine). The 4,4-coupling of DMAP and 2,2-coupling of 9-azajulolidine can be achieved either by the reaction of 2 with L, or by one-electron reduction of borenium cations 5 and 6. Oxidation of 3 and 4 leads to C–C cleavage and regeneration of 5 and 6. All new compounds have been structurally characterized.


 Please wait while we load your content...
Please wait while we load your content...