Synthesis of unsymmetrical ketones by applying visible-light benzophenone/nickel dual catalysis for direct benzylic acylation†
Abstract
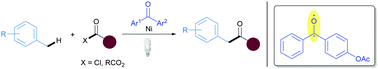
Herein, we report a dual catalytic system for the direct benzylic C–H acylation reaction furnishing a variety of unsymmetrical ketones. A benzophenone-derived photosensitizer combined with a nickel catalyst has been established as the catalytic system. Both acid chlorides and anhydrides are able to acylate the benzylic position of toluene and other methylbenzenes. The method offers a valuable alternative to late transition metal catalyzed C–H acylation reactions.


 Please wait while we load your content...
Please wait while we load your content...