Bifunctional phosphine ligand-enabled gold-catalyzed direct cycloisomerization of alkynyl ketones to 2,5-disubstituted furans†
Abstract
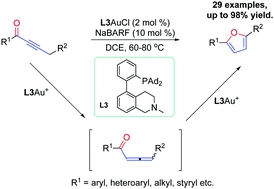
An efficient synthesis of 2,5-disubstituted furans directly from alkynyl ketones has been developed via tandem gold(I)-catalyzed isomerization of alkynyl ketones to allenyl ketones and cycloisomerization. The key to the success of this chemistry is the use of a biphenyl-2-ylphosphine ligand featuring a critical remote tertiary amino group.


 Please wait while we load your content...
Please wait while we load your content...