Concerted proton–electron transfer reactions of manganese–hydroxo and manganese–oxo complexes
Abstract
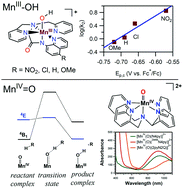
The enzymes manganese superoxide dismutase and manganese lipoxygenase use MnIII–hydroxo centres to mediate proton-coupled electron transfer (PCET) reactions with substrate. As manganese is earth-abundant and inexpensive, manganese catalysts are of interest for synthetic applications. Recent years have seen exciting reports of enantioselective C–H bond oxidation by Mn catalysts supported by aminopyridyl ligands. Such catalysts offer economic and environmentally-friendly alternatives to conventional reagents and catalysts. Mechanistic studies of synthetic catalysts highlight the role of Mn–oxo motifs in attacking substrate C–H bonds, presumably by a concerted proton–electron transfer (CPET) step. (CPET is a sub-class of PCET, where the proton and electron are transferred in the same step.) Knowledge of geometric and electronic influences for CPET reactions of Mn–hydroxo and Mn–oxo adducts enhances our understanding of biological and synthetic manganese centers and informs the design of new catalysts. In this Feature article, we describe kinetic, spectroscopic, and computational studies of MnIII–hydroxo and MnIV–oxo complexes that provide insight into the basis for the CPET reactivity of these species. Systematic perturbations of the ligand environment around MnIII–hydroxo and MnIV–oxo motifs permit elucidation of structure–activity relationships. For MnIII–hydroxo centers, electron-deficient ligands enhance oxidative reactivity. However, ligand perturbations have competing consequences, as changes in the MnIII/II potential, which represents the electron-transfer component for CPET, is offset by compensating changes in the pKa of the MnII–aqua product, which represents the proton-transfer component for CPET. For MnIV–oxo systems, a multi-state reactivity model inspired the development of significantly more reactive complexes. Weakened equatorial donation to the MnIV–oxo unit results in large rate enhancements for C–H bond oxidation and oxygen-atom transfer reactions. These results demonstrate that the local coordination environment can be rationally changed to enhance reactivity of MnIII–hydroxo and MnIV–oxo adducts.


 Please wait while we load your content...
Please wait while we load your content...