Unprecedented reductive cyclisation of salophen ligands to tetrahydroquinoxalines during metal complex formation†
Abstract
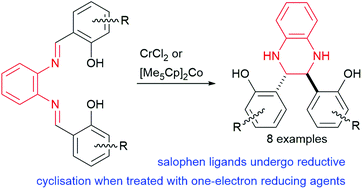
The synthesis of novel tetrahydroquinoxalines by a metal induced one-electron reductive cyclisation of salophen ligands was found to occur when a salophen ligand was treated with chromium(II) chloride or decamethylcobaltocene.


 Please wait while we load your content...
Please wait while we load your content...