Nitrosoarene-catalyzed regioselective aromatic C–H sulfinylation with thiols under aerobic conditions†
Abstract
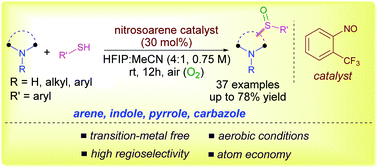
Aromatic amines and (hetero)arenes, such as indoles and pyrroles, are regioselectively sulfinylated under mild aerobic conditions using nitrosoarenes as a redox-catalyst. The nitrosoarene is involved in the electron transfer process with arenes to generate a crucial arene radical cation intermediate for C–H sulfinylation. The present methodology requires no directing group, can be scaled up easily and is applicable for the late-stage functionalization of drug molecules and natural products with high regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...