Binding modes of thioflavin T and Congo red to the fibril structure of amyloid-β(1–42)†
Abstract
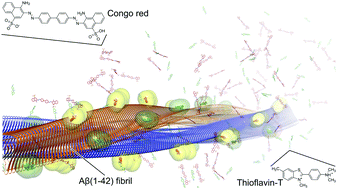
Binding modes for the amyloid-β(1–42) fibril fluorescent dyes thioflavin T and Congo red were predicted by molecular dynamics simulations and binding free energy calculations. Both probes bind on the fibril surface to primarily hydrophobic grooves, with their long axis oriented almost parallel to the fibril axis. The computed binding affinities are in agreement with experimental values. The binding modes also explain observables from previous structural studies and, thus, provide a starting point for the systematic search and design of novel molecules, which may improve in vitro diagnostics for Alzheimer's disease.


 Please wait while we load your content...
Please wait while we load your content...