Electrochemical nickel-catalyzed Migita cross-coupling of 1-thiosugars with aryl, alkenyl and alkynyl bromides†
Abstract
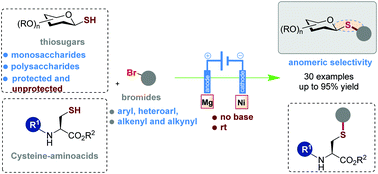
Here we report a simple route towards the synthesis of thioglycosides, in which electrochemical cross-coupling is used to form a S–C glycosidic bond from protected and unprotected thiosugars with functionalized aryl bromides under base free conditions. The reaction manifold that we report here demonstrates the power of electrochemistry to access highly complex glycosides under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...