A new Ni–diaminoglyoxime–g-C3N4 complex towards efficient photocatalytic ethanol splitting via a ligand-to-metal charge transfer (LMCT) mechanism†
Abstract
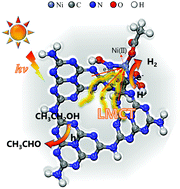
We report a novel Ni–diaminoglyoxime–g-C3N4 (Ni–DAG–CN) complex for H2 evolution through photocatalytic ethanol splitting. Compared to that of pristine g-C3N4, Ni–DAG–CN exhibits a 21-fold enhancement of photocatalytic activity (296.1 μmol h−1 g−1) under irradiation with excellent stability. The enhanced photocatalytic activity can be attributed to a proposed ligand-to-metal charge transfer (LMCT) mechanism, which is illustrated both experimentally and theoretically. This work provides great potential in the future design of low-cost, high-performance photocatalysts for H2 evolution from alcohol splitting.


 Please wait while we load your content...
Please wait while we load your content...