On-surface synthesis of planar acenes via regioselective aryl–aryl coupling†
Abstract
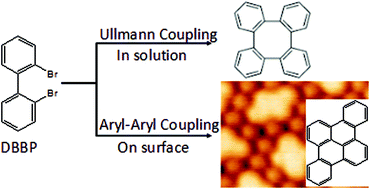
The reaction of 2,2′-dibromo-biphenyl on a Ag(111) surface leads to the formation of planar acenes with a high-regioselectivity rather than nonplanar saddle-shaped tetraphenylene as the typical product in solution chemistry. The regioselective aryl–aryl coupling reaction is attributed to the hydrogen repulsion between the reactants on the confined two-dimensional surface.


 Please wait while we load your content...
Please wait while we load your content...