β-Silicon-effect-promoted intermolecular site-selective C(sp3)–H amination with dirhodium nitrenes†
Abstract
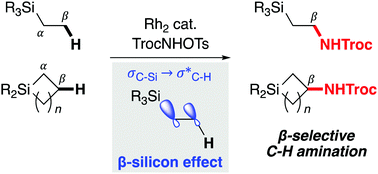
A dirhodium-catalyzed, β-selective C–H amination of organosilicon compounds has been developed. Primary C(sp3)–H bonds of silylethyl groups and secondary C(sp3)–H bonds of silacycloalkanes can be selectively converted to C–N bonds at the β-position of the silicon atoms. The experimental data and theoretical calculations indicate that the strong σ-donor ability of the carbon–silicon bonds is responsible for the β-selectivity. Kinetic isotope effects clearly demonstrate that the C–H bond cleavage step is not turnover-limiting, but selectivity-determining.


 Please wait while we load your content...
Please wait while we load your content...