Dual ligand-promoted palladium-catalyzed nondirected C–H alkenylation of aryl ethers†
Abstract
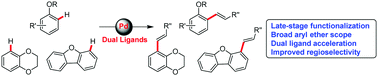
Direct C–H functionalization of aryl ethers remains challenging owing to their low reactivity and selectivity. Herein, a novel strategy for nondirected C–H alkenylation of aryl ethers promoted by a dual ligand catalyst was demonstrated. This catalytic system readily achieved the highly efficient alkenylation of alkyl aryl ethers (anisole, phenetole, n-propyl phenyl ether, n-butyl phenyl ether and benzyl phenyl ether), cyclic aryl ethers (1,4-benzodioxan, 2,3-dihydrobenzofuran, dibenzofuran), and diphenyl oxides. Moreover, the proposed methodology was successfully employed for the late-stage modification of complex drugs containing the aryl ether motif. Interestingly, the compounds developed herein displayed fluorescent properties, which would facilitate their biological applications.


 Please wait while we load your content...
Please wait while we load your content...