Cascade reaction of isatins with nitro-substituted enamines: highly selective synthesis of functionalized (Z)-3-(1-(arylamino)-2-oxoarylidene)indolin-2-ones†
Abstract
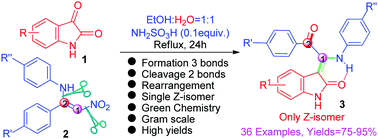
A novel protocol for the construction of functionalized (Z)-3-(1(-arylamino)-2-oxoarylidene)indolin-2-ones (AOIDOs) from isatins 1 with nitro-substituted enamines 2via an unprecedented cascade reaction catalyzed by sulfamic acid is developed. The AOIDOs are prepared by simply refluxing a mixture of isatins with a wide variety of nitro-substituted enamines. Interestingly, the AOIDOs 3 were formed through the novel cascade reaction involving a unique cleavage of two C–N bonds of the nitro-substituted enamines. Overall, the novel reaction is accomplished by the formation of three new bonds and the cleavage of two C–N bonds in a single step. This protocol can be used in the synthesis of a wide variety of AOIDOs and is suitable for combinatorial and parallel syntheses of natural-like AOIDO products.


 Please wait while we load your content...
Please wait while we load your content...