A highly substituted pyrazinophane generated from a quinoidal system via a cascade reaction†
Abstract
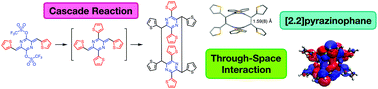
The generation of a highly-substituted [2.2](2,5)pyrazinophane via a cascade reaction is presented. The pyrazinophane product is formed via the dimerization of a member of the para-azaquinodimethane (p-AQM) family of conjugated quinoidal compounds—reactivity that sheds light on the nature of stability in p-AQMs. Additionally, the electronic and structural nature of this highly-strained ring system are characterized.


 Please wait while we load your content...
Please wait while we load your content...