Palladium-catalyzed intermolecular C–H silylation initiated by aminopalladation†
Abstract
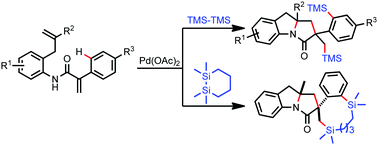
A Pd(II)-catalyzed intermolecular C–H silylation reaction initiated by aminopalladation has been developed. The C–H bonds were activated by an alkyl Pd(II) species generated through aminopalladation and then disilylated with hexamethyldisilane to form disilylated indolines as the final products. The reaction provides a new method for the introduction of silyl groups into complex organic molecules.


 Please wait while we load your content...
Please wait while we load your content...