Hydrogen order at the surface of ice Ih revealed by vibrational spectroscopy†
Abstract
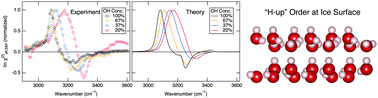
Among numerous crystalline phases of ice, the Ih phase is the most stable above 72 K at atmospheric pressure. It is well established that the orientations of water molecules in the bulk of ice Ih are statistical without long-range order. However, the orientational order of water at the surface of ice Ih has been enigmatic. Here we show that the surface of ice Ih at 100 K has hydrogen order with the OH group pointing upward to the air (“H-up” orientation). We applied nonlinear optical spectroscopy and theoretical modeling to the surface of isotopically pure and diluted ice Ih and observed OH stretch vibrational signatures attributed to H-up ordering. Furthermore, we found that this hydrogen order takes place despite a more inhomogeneous microenvironment at the surface than in the bulk. Our results suggest the prominent role of the surface to allow the reorientation of water molecules for hydrogen ordering that is virtually prohibited in the bulk.


 Please wait while we load your content...
Please wait while we load your content...