Short and modular synthesis of tetraarylsalicylaldehydes†
Abstract
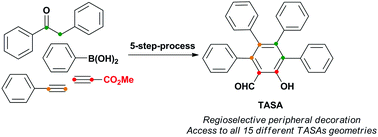
In this study, we describe a novel strategy that allows the obtention of all 15 possible substitution geometries of perarylated salicylaldehydes with total control of the regioselectivity. This strategy entitles the formation of the salicylaldehyde core via a Claisen rearrangement of propargyl vinyl ethers, followed by bromination and Pd-catalyzed aryl–aryl cross-coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...