Halohydroxylation of alkenyl MIDA boronates: switchable stereoselectivity induced by B(MIDA) substituent†
Abstract
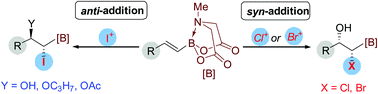
The synthesis of α-boryl halohydrins via difunctionalization of alkenyl MIDA boronates has been reported. Intriguing stereoselectivity was found with different halogen sources, which arises from the special stabilizing effect of the B(MIDA) moiety. The transformation provided cis addition products using Cl+ or Br+ as the halogen source, while trans addition products were obtained when I+ was employed.


 Please wait while we load your content...
Please wait while we load your content...