para-Selective arylation and alkenylation of monosubstituted arenes using thianthrene S-oxide as a transient mediator†
Abstract
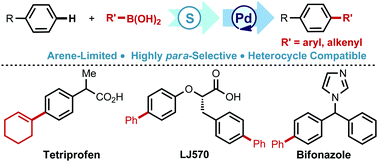
Using thianthrene S-oxide (TTSO) as a transient mediator, para-arylation and alkenylation of mono-substituted arenes have been demonstrated via a para-selective thianthrenation/Pd-catalyzed thio-Suzuki–Miyaura coupling sequence under mild conditions. This reaction features a broad substrate scope, and functional group and heterocycle tolerance. The versatility of this approach was further demonstrated by late-stage functionalization of complex bioactive scaffolds, and direct synthesis of some pharmaceuticals, including Tetriprofen, Ibuprofen, Bifonazole, and LJ570.


 Please wait while we load your content...
Please wait while we load your content...