Lateral lithiation in deep eutectic solvents: regioselective functionalization of substituted toluene derivatives†
Abstract
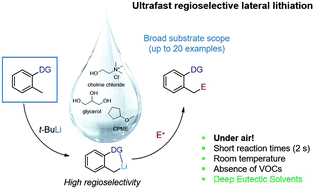
The heteroatom-directed lateral lithiation of functionalized toluenes in a choline chloride-based eutectic mixture is reported. The metalations proceed within ultrafast reaction times, with a broad substrate and electrophile scope. The directing groups provide a rapid and high regioselective access to functionalized aromatic derivatives of remarkable synthetic value.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...