Nickel-catalyzed intramolecular desymmetrization addition of aryl halides to 1,3-diketones†
Abstract
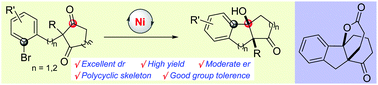
A nickel-catalyzed intramolecular addition of aryl halides to 1,3-diketones was first developed. This desymmetrization reaction afforded polycyclic products bearing two tetrasubstituted centers with excellent diastereoselectivities and high yields. Moderate enantioselectivities were achieved in the presence of a chiral ligand. This transformation has great potential for the synthesis of polycyclic compounds including spiro[4,4,3,0] compounds.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...