Construction of sequence-defined polytriazoles by IrAAC and CuAAC reactions†
Abstract
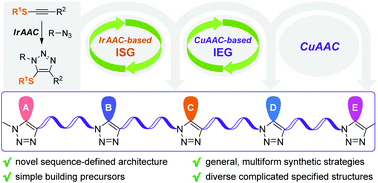
Here we report the first synthesis of sequence-defined polytriazoles, in which different side groups are sequentially anchored to the C-5 position of 1,2,3-triazole rings. By using efficient synthetic strategies based on IrAAC and CuAAC, different monodispersed polytriazoles with up to ∼5.3 kDa and 31-mer were constructed. Structural characterization via NMR, SEC, MALDI-TOF-MS, tandem MS and FTICR-MS evidenced the formation of polytriazoles with the desired specified sequences and exact chain lengths.


 Please wait while we load your content...
Please wait while we load your content...