N-Heterocyclic carbene (NHC)-catalyzed tandem imine umpolung–aza-Michael addition–oxidation of β-carboline cyclic imines†
Abstract
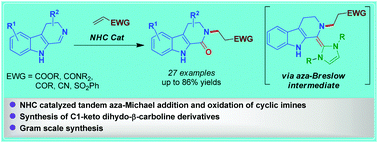
Herein we describe an NHC-catalyzed umpolung of β-carboline-based cyclic imines for their conversion to the corresponding N-substituted cyclic amides. The key to the success of this transformation appears to be that NHC upon reaction with the imine concomitantly goes through the generation of an aza-Breslow intermediate and aza-Michael addition followed by oxidation with molecular oxygen to deliver the N-substituted amide products. The developed method has enabled the synthesis of various biologically relevant β-carboline-1-one derivatives in good yields.


 Please wait while we load your content...
Please wait while we load your content...