Manipulating regioselectivity of an epoxide hydrolase for single enzymatic synthesis of (R)-1,2-diols from racemic epoxides†
Abstract
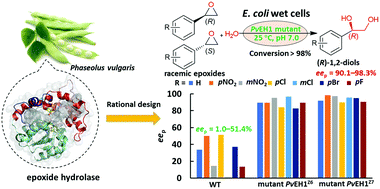
Both the activity and regioselectivity of Phaseolus vulgaris epoxide hydrolase were remarkably improved via reshaping two substrate tunnels based on rational design. The elegant one-step enantioconvergent hydrolysis of seven rac-epoxides was achieved by single mutants, allowing green and efficient access to valuable (R)-1,2 diols with high eep (90.1–98.3%) and yields.


 Please wait while we load your content...
Please wait while we load your content...