Arylation and alkenylation of activated alkyl halides using sulfonamides†
Abstract
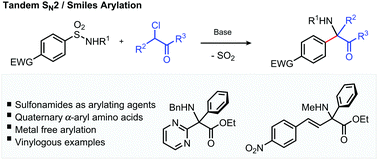
A variety of quaternary aryl amino acid derivatives can be synthesised using tandem SN2/Smiles rearrangement chemistry involving aryl sulfonamides and α-chloro carbonyl compounds. The reaction harnesses a sulfur dioxide extrusion pathway to construct a C–N and C–Caryl bond under simple conditions with no requirement for organometallics or transition metal catalysts. The reaction is also successful for alkenyl sulfonamides, producing sterically congested quaternary alkene amino acid derivatives.


 Please wait while we load your content...
Please wait while we load your content...