Diamine-mediated degradative dimerisation of Morita–Baylis–Hillman ketones†
Abstract
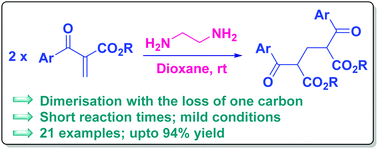
A degradative dimerisation of Morita–Baylis–Hillman ketones was observed in the presence of a primary diamine. The reaction proceeded swiftly to produce methylene-bridged 1,3-dicarbonyl compounds. A brief mechanistic investigation alluded to a retro-Mannich reaction as the key step of the transformation.


 Please wait while we load your content...
Please wait while we load your content...