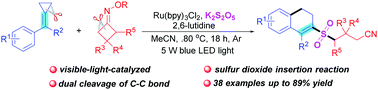
Visible-light photoredox-catalyzed dual C–C bond cleavage: synthesis of 2-cyanoalkylsulfonylated 3,4-dihydronaphthalenes through the insertion of sulfur dioxide†
Abstract
An efficient novel visible-light photoredox-catalyzed dual carbon–carbon bond cleavage of methylenecyclopropanes and cycloketone oximes for the synthesis of 2-cyanoalkylsulfonated 3,4-dihydronaphthalenes through the insertion of sulfur dioxide is established. This dual cleavage of carbon–carbon bonds involves a radical pathway and goes through a sequence of iminyl radical formation, carbon–carbon bond cleavage, sulfur dioxide insertion, sulfonyl radical addition, another carbon–carbon bond cleavage, and intramolecular cyclization.


 Please wait while we load your content...
Please wait while we load your content...