Iron(ii)-chloride-catalyzed regioselective azidation of allenamides with TMSN3†
Abstract
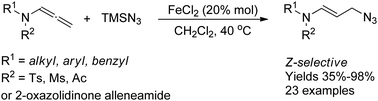
In this study, we developed a new cost-effective and efficient route for accessing allyl azides: iron(II)-chloride-catalyzed regioselective azidation of allenamides with TMSN3. This process is highly regio- and stereoselective, affording (E)-allyl azides in good to excellent yields. Due to the versatility of the azide group, these products can be transformed in situ to allyl triazoles and allyl amines, facilitating numerous subsequent chemical modifications.


 Please wait while we load your content...
Please wait while we load your content...