Stereoselective synthesis of fully substituted ethylenes via an Ag-catalyzed 1,6-nucleophilic addition/annulation cascade†
Abstract
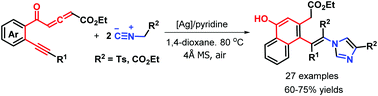
A catalytic 1,6-nucleophilic addition/annulation cascade was developed for the first time, and used to produce 27 hitherto unreported ethylene-linked 1-naphthol-imidazole pairs with generally good yields and complete stereoselectivity. An Ag2O-catalyzed reaction of yne-allenone esters with tosylmethyl isocyanide proceeded efficiently, and provided a simple and convergent protocol for the synthesis of fully substituted (Z)-ethylenes whereas tetrasubstituted (E)-ethylenes were obtained when ethyl isocyanoacetate was employed in this transformation. The reaction pathway consists of [2+2] cycloaddition, 1,6-nucleophilic addition and [3+2] cycloaddition, leading to continuous multiple bond-forming events including C–C and C–N bonds to construct complex molecules.


 Please wait while we load your content...
Please wait while we load your content...