Oxidation of an indole substrate by porphyrin iron(iii) superoxide: relevance to indoleamine and tryptophan 2,3-dioxygenases†
Abstract
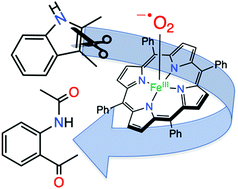
Reaction of FeIII(O2˙−)(TPP) with 2,3-dimethylindole at −40 °C gives the ring-opened, dioxygenated N-(2-acetyl-phenyl)-acetamide product. The reaction was monitored in situ by low-temperature UV-vis and 1H NMR spectroscopies. This work demonstrates that a discrete iron(III)(superoxo) porphyrin is competent to carry out indole oxidation, as proposed for the tryptophan and indoleamine 2,3-dioxygenases.


 Please wait while we load your content...
Please wait while we load your content...