Visible-light-mediated arylation of ortho-hydroxyarylenaminones: direct access to isoflavones†
Abstract
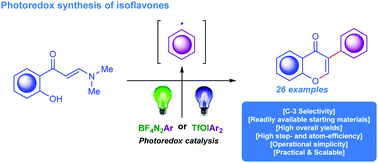
In this work we highlight two new methods for the synthesis of isoflavones through consecutive domino arylation of ortho-hydroxyarylenaminones with in situ photogenerated aryl radicals. As precursors for aryl radicals we used aryl onium reagents such as diazonium and diaryliodonium salts. Notably, the photo-Meerwein arylation by aryl diazonium tetrafluoroborates demonstrated high efficiency in terms of yields and can be considered as a method of choice for the straightforward assembly of 3-aryl-substituted chromones. Ultimately, 26 compounds were prepared in good to excellent yields using the developed synthetic protocols.
- This article is part of the themed collection: Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...