Ruthenium-catalyzed benzylic substitution of benzyl esters with stabilized carbon nucleophiles†
Abstract
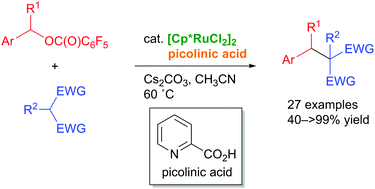
We have accomplished the ruthenium-catalyzed benzylic substitution of benzyl esters with a stabilized carbon nucleophile. A [Cp*RuCl2]2/picolinic acid catalyst system promoted the reaction of 2-naphthylmethyl-2,3,4,5,6-pentafluorobenzoates with a series of stabilized carbon nucleophiles such as malonates, β-ketoesters, and diketones to give the corresponding benzylic alkylation products in moderate to high yields. We proposed a plausible reaction mechanism that could involve a (π-benzyl)ruthenium intermediate.


 Please wait while we load your content...
Please wait while we load your content...