Nickel-catalyzed carbodifunctionalization of N-vinylamides enables access to γ-amino acids†
Abstract
A nickel-catalyzed tandem reaction of N-vinylamides with arylboronic acids and bromodifluoroacetate has been developed. The use of amide carbonyl as a chelating group efficiently furnishes a series of protected α,α-difluoro-γ-amino acid esters. The reaction can also extend to bromoacetate and 2-bromomalonate. The advantages of this protocol are high functional group tolerance and a broad substrate scope, including a variety of N-vinylamides. In particular, the use of removable amide carbonyl groups provides potential opportunities for applications in peptide chemistry and protein engineering.
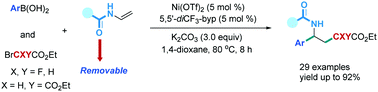


 Please wait while we load your content...
Please wait while we load your content...