Synthesis and characterization of high affinity fluorogenic α-synuclein probes†
Abstract
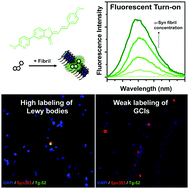
Fluorescent small molecules are powerful tools for imaging α-synuclein pathology in vitro and in vivo. In this work, we explore benzofuranone as a potential scaffold for the design of fluorescent α-synuclein probes. These compounds have high affinity for α-synuclein, show fluorescent turn-on upon binding to fibrils, and display different binding to Lewy bodies, Lewy neurites and glial cytoplasmic inclusion pathologies in post-mortem brain tissue. These studies not only reveal the potential of benzofuranone compounds as α-synuclein specific fluorescent probes, but also have implications for the ways in which α-synucleinopathies are conformationally different and display distinct small molecule binding sites.


 Please wait while we load your content...
Please wait while we load your content...