Synthesis of difluoromethyl and deuterium-labeled difluoromethyl thioethers from aliphatic electrophiles†
Abstract
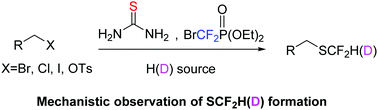
A one-pot difluoromethylthiolation of alkyl electrophiles with thiourea and diethyl bromodifluoromethylphosphonate is described. The transition-metal-free approach, readily available reagents, and mild conditions provide a practical way for the synthesis of difluoromethyl thioethers. By changing the “H” source to the most commonly used “D” sources CD3OD and D2O, this strategy enables efficient synthesis of SCF2D-substituted molecules in good yields with high levels of D incorporation.


 Please wait while we load your content...
Please wait while we load your content...