Cu(i)-Catalyzed asymmetric intramolecular addition of aryl pinacolboronic esters to unactivated ketones: enantioselective synthesis of 2,3-dihydrobenzofuran-3-ol derivatives†
Abstract
An (S,S)-QuinoxP*-supported Cu(I) catalyst has been disclosed for highly enantioselective intramolecular addition of aryl pinacolboronic esters to unactivated ketones under mild reaction conditions. This protocol showcases a broad substrate tolerance and allows access to various chiral 2,3-dihydrobenzofuran-3-ol derivatives in generally good yields with excellent enantioselectivities.
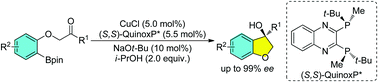


 Please wait while we load your content...
Please wait while we load your content...