An organocatalytic asymmetric Friedel–Crafts reaction of 2-substituted indoles with aldehydes: enantioselective synthesis of α-hydroxyl ketones by low loading of chiral phosphoric acid†
Abstract
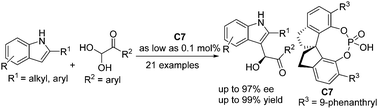
Hydroxyl alkylation of indoles by Friedel–Crafts reaction with a carbonyl compound is a useful strategy. However, the reaction was restricted to ketones due to the easy formation of a bisindole byproduct. Therefore, hydroxyl alkylation of an aldehyde with indole is confronted with great challenges. Here, we report an efficient strategy for asymmetric hydroxyl alkylation of 2-substituted indoles with aldehydes under 0.1 mol% chiral phosphoric acid. A series of α-hydroxyl ketones were obtained in high yields (up to 99%) and good enantioselectivities (up to 97%).


 Please wait while we load your content...
Please wait while we load your content...