The regioselective annulation of alkylidenecyclopropanes by Rh(iii)-catalyzed C–H/C–C activation to access spirocyclic benzosultams†
Abstract
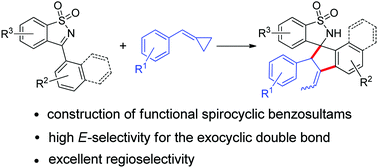
Functionalized benzosultams are an essential class of structural motif found in various biologically active molecules. The synthesis of spirocyclic benzosultams from N-sulfonyl ketimine and alkylidenecyclopropanes (ACPs) under the catalysis of Rh(III) has been developed. This transformation enables the formation of two C–C bonds and a double bond with high E-selectivity through C–H and C–C bond activation. This reaction proceeded smoothly with excellent regioselectivity, high efficiency, and tolerance for various functional groups.


 Please wait while we load your content...
Please wait while we load your content...