Palladium(ii)-catalyzed vinylic geminal double C–H activation and alkyne annulation reaction: synthesis of pentafulvenes†
Abstract
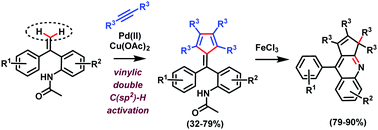
The first transition-metal-catalyzed vinylic geminal double C(sp2)–H activation and di-substituted alkyne annulation reaction is reported. This palladium(II)-catalyzed, amide directed reaction of vinylic compounds with di-substituted alkynes offers an efficient synthetic path to pentafulvenes, which are very important compounds because of their bioactivity and interesting optical properties. A FeCl3-mediated transformation of pentafulvenes to fluorescent cyclopenta[b]quinolines is also developed.


 Please wait while we load your content...
Please wait while we load your content...