Iron-catalyzed stereospecific arylation of enol tosylates using Grignard reagents†
Abstract
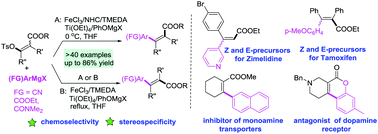
The stereospecific Fe-catalyzed arylation of enol tosylates was reported. Various tri- or tetrasubstituted Z or E-enol tosylates of β-keto esters were arylated using common and Knochel-type Grignard reagents with complete stereofidelity. The precursors for Z/E-zimelidine, tamoxifen and other bioactive compounds were facilely prepared without precious and toxic transition metals.


 Please wait while we load your content...
Please wait while we load your content...